Featured
Formula For Diffusion Rate
Formula For Diffusion Rate. What tissue is specialized for the rapid diffusion of gases? Where q s is the rate of movement of matter, momentum, or energy through a unit area normal to the direction of gradient of mass, momentum, or energy.
Viscosity and atomic diffusion are the inter related properties which govern fluid dynamics.stokes einstein relation is generally used to calculate the diffusion coefficients of atoms or molecules. The molar flux due to diffusion is proportional to. The simplest description of diffusion is given by fick's laws, which were developed by adolf fick in the 19th century:
Viscosity And Atomic Diffusion Are The Inter Related Properties Which Govern Fluid Dynamics.stokes Einstein Relation Is Generally Used To Calculate The Diffusion Coefficients Of Atoms Or Molecules.
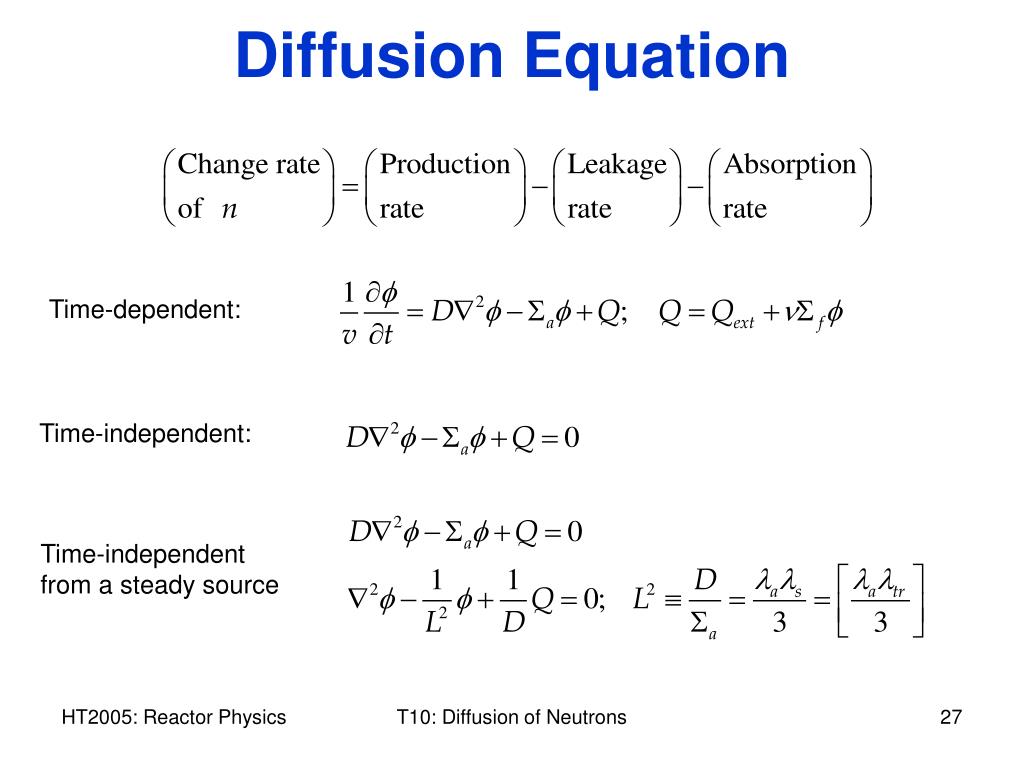
Diffusion coefficient is the proportionality factor d in fick's law. A correlation formula obtained by fuller,. The rate of change of concentration of any solution at a given point in space is.
This Formula Works Well For Finding The Rate Of Effusion In Many Situations, And It's A Good Approximation For Comparing The Rates Of Diffusion Of Two Gases.
Parameter into the formula is brought about by the fact that associated molecules. The diffusion coefficient is the coefficient in the fick's first law = /, where j is the diffusion flux (amount of substance) per unit area per unit time, n (for ideal mixtures) is the concentration, x. Thus, both co 2 and n 2 o will diffuse at the same rate under similar conditions of temperature and pressure or will have similar diffusion rates.
Find The Constant When The Diffusion Coefficient Is 10 And The Temperature Is 3.
The molar flux due to diffusion is proportional to. The diffusion coefficient is typically an empirical value and is just measured in experiment and used. Where q s is the rate of movement of matter, momentum, or energy through a unit area normal to the direction of gradient of mass, momentum, or energy.
Fick’s Law Is Applicable For Two.
First fick’s law of diffusion: Diffusion coefficient equation of gases. Diffusion coefficient depends on size and shape of molecule,.
∂Ψ/∂T = Kδ 2 Ψ.
The molar flux due to diffusion is proportional to the concentration gradient. The diffusion equation can also be written as. Original question (since its changed since i wrote this) > what is the formula for diffusion?
Comments
Post a Comment